-
Plot no: 3541 GIDC Phase – IV, Chhatral Ta: Kalol Dist: Gandhinagar Gujarat

Years of
Experiment with the best lab products and service
We are AARPAN REMEDIES LLP, a contract research and
manufacturing service provider for the field of pharmaceutical
industry. Dedicatedly working as manufacturing partner to
various pharmaceutical companies, working actively on various
intermediates and API for the customers.
Established as a contract research & manufacturing services
company, having GMP manufacturing unit at chhatral, Gujarat.
Company focused as a research and development company,
focused on developing products for its customer from R&D level
to validation and finally commercial production
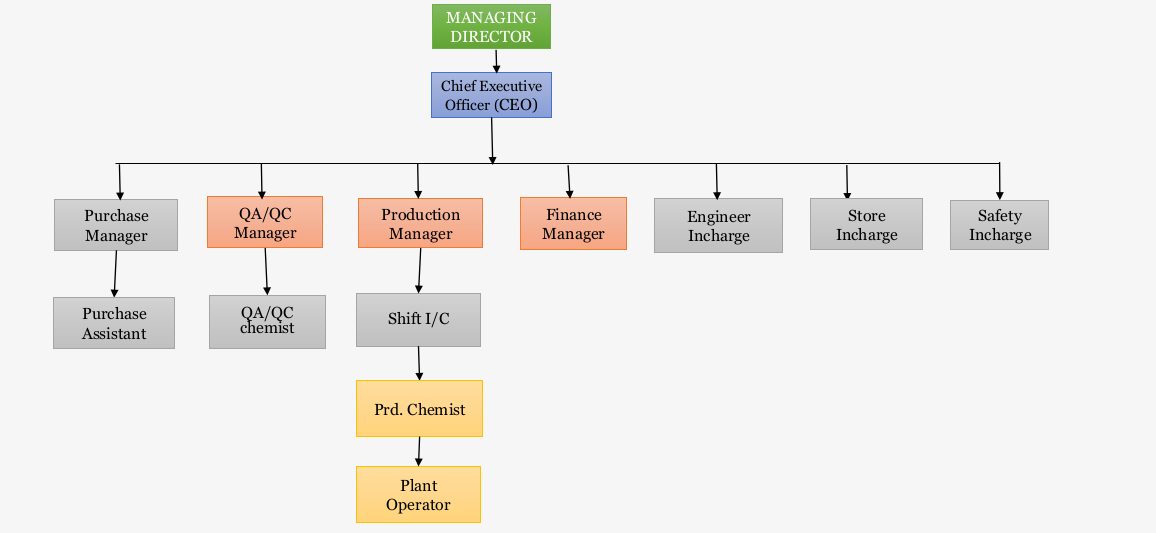
OPERATIONS
The manufacturing site is strategically designed/installed/operated as Contract research and
manufacturing service provider. Site is operational since 2021. The plant is designed as per
the cGMP norms for validating/manufacturing any intermediate/API/novel molecule in best
of manufacturing standards, our plant is operated as per the ICHQ7 guidelines for
manufacturing of API. Having Pilot plant (controlled area)/ Intermediate section/Clean room
section. The QC also includes all the modern equipment required for API manufacturing.

Since
2021Agriculture
The great explorers the truth the claims of duty.
Environmental
Holds in these matters to this principle of selection.
Pharmaceutical
Great pleasures or else cases endures pains avoid.
Industries that we served
Food Solutions
The great explorers the truth the claims of duty.
Automotive
Holds in these matters to this principle of selection.
Manufacturing Area Design
Plant-1
Ground floor Area :279.12 Sq.Mtrs
First floor Area :279.12 Sq.Mtrs
Second floor Area :279.12 Sq.Mtrs
Plant-1
Ground floor Area :279.12 Sq.Mtrs
First floor Area :279.12 Sq.Mtrs
Second floor Area :279.12 Sq.Mtrs
Plant-2
Ground floor Area :41.2 Sq.Mtrs
First floor Area :41.2 Sq.Mtrs
Plant-2
Ground floor Area :41.2 Sq.Mtrs
First floor Area :41.2 Sq.Mtrs
Plant-3
Second floor Area :41.2 Sq.Mtrs
Plant-3
Second floor Area :41.2 Sq.Mtrs
Legal & Statutory compliance
- Maintain organizational integrity and mitigate risks through meticulous adherence to legal and statutory requirements.
- Safeguarding against potential liabilities and ensuring a foundation of trust and accountability.
Continuous Earth Monitoring System
- To Enhanced safety for personnel and equipment by Early detection of earthing Discontinuity to minimizing static incident, We have installed Earthing Monitoring System for 10 Equipment’s. and another one system under Installation.
- It having monitoring capabilities of Continuous earth resistance and continuity and gives real-time alerts.

Material & Solvent handling Practice
- We are following Standard Procedure for Raw material and finished product Identification & Labelling, Handling and Storage.
- We have a Separate Ware-house From Company Premises to Store the Material and Solvent.
- Well Defined SOP For Solvent handling and Storage as per their Compatibility.
Manufacturer And Dispatch
- We Processed Work in Flash column system for Isomer separation,
- We also working on Phytochemicals purification and concentration enrichment by Column Chromatography at Lab Scale Trials.
| Year | Input | Dispatched | Solvent Handle |
|---|---|---|---|
| 2022 | 4200 kg | 3400 Kg | 6,50,000 Ltr. |
| 2023 | 1650 Kg | 1300 Kg | 2,50,000 Ltr. |
| 2024 | 4500 Kg (Excepted) |
Quality Policy-EHS Policy
- Our Quality and EHS policies prioritize continuous improvement and compliance, ensuring the highest standards of product quality and environmental health and safety across all operations.
- Ensuring the Quality and EHS is our priority, as reflected in our comprehensive Policy, which outlines our commitment to excellence.
- Well defined and established Risk Assessment system Like SWOT Analysis & Context analysis & Risk assessment.
- Recently EHS system is audited by our customers Dishman Carbogen Amcis Ltd. And Torrent Pharmaceuticals Ltd.
- Our Quality Policy align With ICH Q7 Guideline for good manufacturing practice for the manufacturing of active pharmaceutical ingredients (APIs).
- 20 floor persons are Trained & Certified from Fire institutes in “FIRE & SAFETY” Programme.
KEY STRENGTH
At Aarpan Remedies LLP, we specialize in contract research and manufacturing for the pharmaceutical industry, offering end-to-end solutions from R&D to commercial production. Our GMP-certified manufacturing facility in Chhatral, Gujarat, ensures compliance with global quality standards, delivering high-quality pharmaceutical intermediates and APIs. With a strong R&D-driven approach, we focus on innovation, process optimization, and rapid scale-up, enabling seamless technology transfer and cost-effective manufacturing. Our customer-centric approach ensures tailored solutions, while our commitment to sustainability and ethical practices drives responsible manufacturing. By integrating cutting-edge technology, stringent quality control, and regulatory compliance, we strive to be a trusted partner for pharmaceutical companies worldwide.
Safety by design manufacturing site
- Plant designed keeping safety first in the design concept. Having well ventilated equipment and process area, well designed escape route in case of any Emergency.
Zero carbon footprint mission
- Being responsible to beloved mother nature and with our working standards of being conscious of our existence, we will be Zero carbon foot print company in next 10 years, operating at carbon neutrality via Tree plantation and energy generation via waste. Will be planting more than 2 millions plants in the surrounding community.
Experience of CRO
- With the experience of CRO of almost 10 years, We are aware of the critical requirements of the customer.
APPROVALS
- Environment Clearance for the project from state pollution control board
- No objection Certificate from state pollution control board
- FDA license from food and drugs department for manufacturing of API
- FIRE NOC from fire & Safety Department
- License for usage of various solvents from regulatory authorities
Certifications
- ISO 9001: Quality Management system
- ISO 14001: Environment Health & Safety
- ISO 18001: Occupational Health and Safety Assessment
QUALITY ASSURANCE
- Well established quality assurance system as per ICH Q7 guidelines.
- Elaborate list of Standard operating procedure for all components of the operations.
- Proper training and record on SOP/Format filling/Safety Etc.
QUALITY CONTROL
| Equipment | Details |
|---|---|
| HPLC | Simadzu |
| Gas Chromatography | Simadzu |
| Balances | Simadzu |
| Ph meter | Not Specified |
| Kf | Not Specified |
| Melting point apparatus | Not Specified |
EQUIPMENT LIST (Pilot Plant – 500 Gms to 10 Kg)
| Equipment / reactor | Size |
|---|---|
| Glass assembly with addition/Receiver/Collection/Heating/Chilling | 100 Ltr |
| Glass assembly | 200 Ltr |
| Glass assembly | 50 Ltr |
| Glass assembly | 50 Ltr |
| CF-SS 316 | 18 " |
| Dryer Cum Oven | 10 Trays |
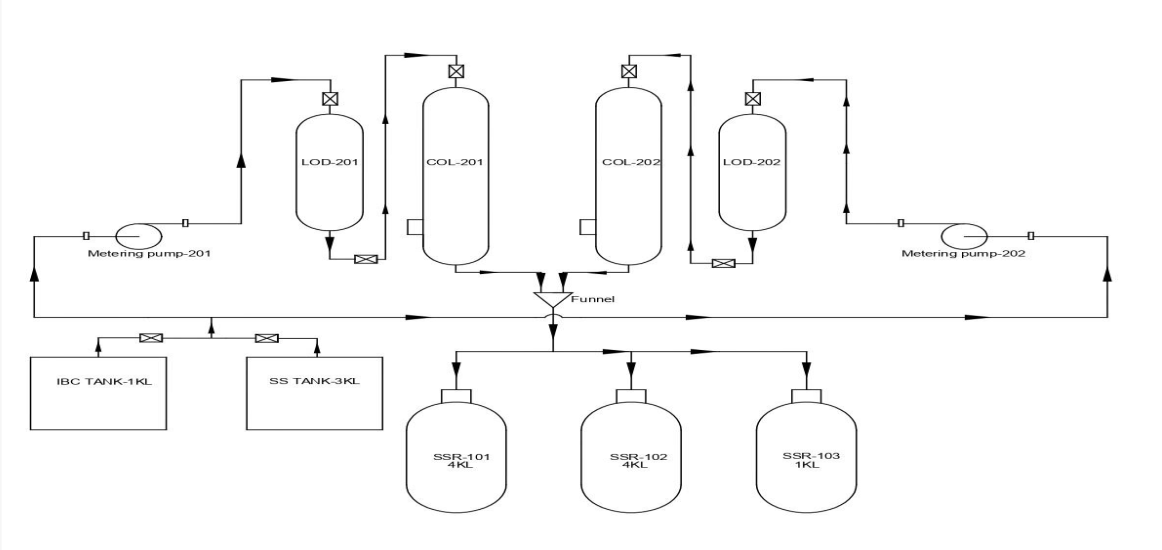
EQUIPMENT LIST (Intermediate Area)
| Equipment / reactor | Size |
|---|---|
| Glass Lined Reactor with Column condenser | 3 KL |
| SS-316 with Column condenser | 4 KL |
| SS-316 with Column condenser | 4 KL |
| SS-316 (High Vacuum / High Temprature) | 1 KL |
| CF - SS 316 | 48" |
| CF - SS 316 | 48" |
| RCVD | 750 Ltrs |
EQUIPMENT LIST (Clean Room Area)
| Equipment / reactor | Size |
|---|---|
| Glass Lined Reactor with Column condenser | 1 KL |
| Glass Lined Reactor with Column condenser | 1 KL |
| SS - 316 | 500 Ltrs |
| SS - 316 | 500 Ltrs |
| CF - SS 316 | 36" |
| VTD | 48 Trays |
OTHER DETAILS
- Strong R&D Team for handling of any type pf chemistry and scale up.
- Strategic partnership with outside quality control lab (USFDA approved) for third party testing of every final material manufactured.
- Having all Govt. as well regulatory compliance for running a chemical company.
Customer’s Visit & Audit
- Torrent Pharmaceuticals Ltd
- Zydus Lifescience
- Bodal Chemicals Ltd
- Azafran Innovacion
- Mack Pharmaceutical
- Piramal Pharmaceuticals
- Others …..